- cross-posted to:
- [email protected]
- cross-posted to:
- [email protected]
Scientists have discovered that the molecule DIM reduces biofilms causing dental plaque by 90%. Its addition to toothpaste and mouthwash could revolutionize dental hygiene. 3,3′-Diindolylmethane (DIM) decreased the Streptococcus mutans biofilm, a leading contributor to plaque and cavities, by 90%.
A significant portion of the global population experiences persistent issues with dental plaque and cavities or will face them at some time. While toothpaste, mouthwash, and routine dental visits help in prevention, there’s always room for improvement.
Researchers from Ben-Gurion University of the Negev, in collaboration with teams from Sichuan University and the National University of Singapore, have identified that 3,3′-Diindolylmethane (DIM) – a naturally occurring molecule also referred to as bisindole – can reduce biofilms responsible for plaque and cavities by a remarkable 90%.


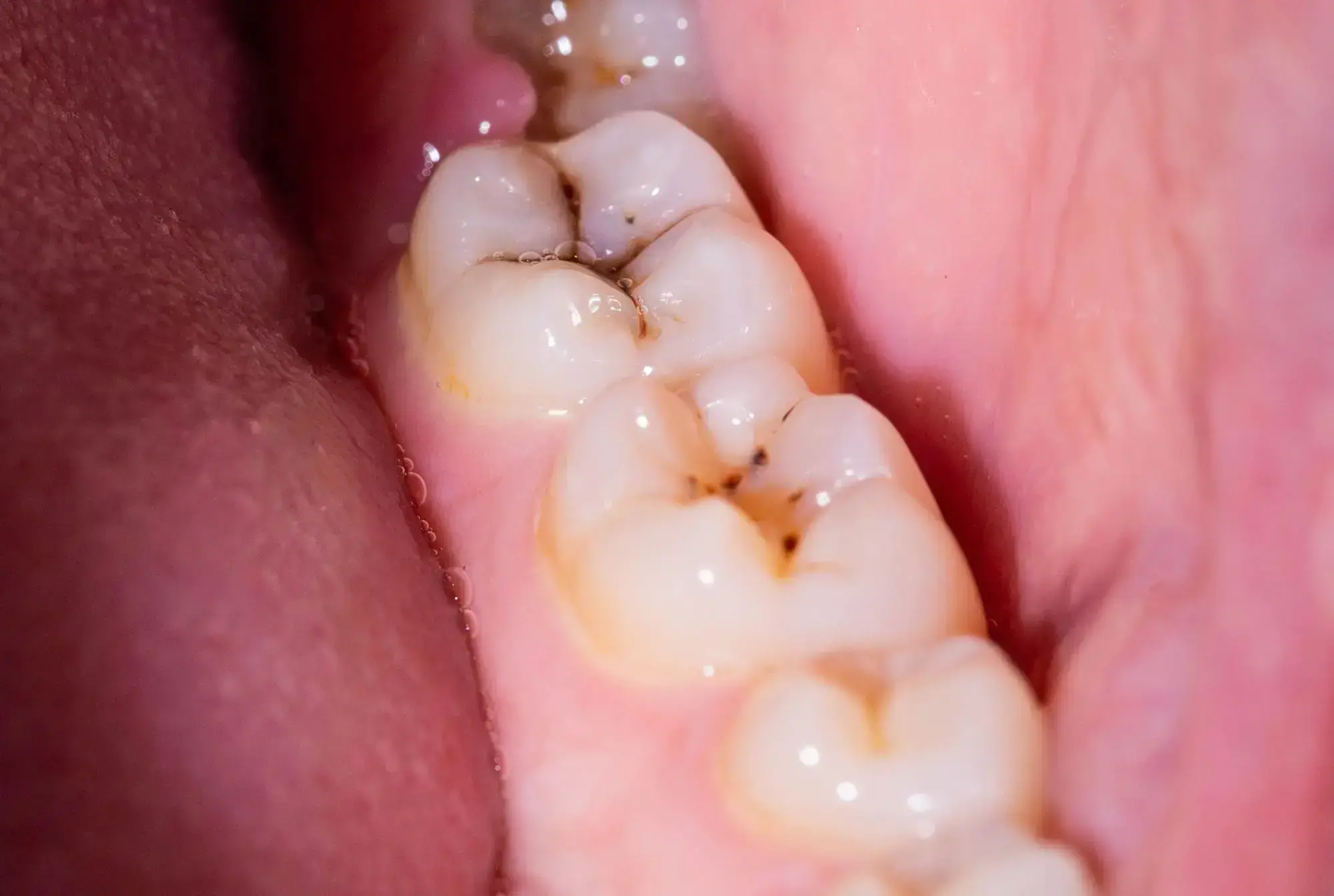
That’s the cool part about chemistry, when used in this synthesis the dichloromethane becomes a whole new (presumably safe, but we’ll see what further testing turns up) molecule. Sodium is explosive and Chlorine is highly corrosive, but combine them and you get regular old table salt. Just because a reagent is dangerous doesn’t mean the products it creates will be.
Edit: I just did a little research and it looks like 3,3′-Diindolylmethane (DIM) is safe for human consumption. It’s already sold as a dietary supplement. It’s marketed to help with metabolism of estrogens but we all know how “trustworthy” the dietary supplement market is.
I don’t think they were implying that DIM is unsafe, but rather saying that they hope people don’t try to handle the dangerous reagent in their backyard.
I understand that. My concern was with amateur chemists mucking around with the dangerous reagent in their backyards. Imagine them ventilating their lab and killing the neighbours dog, things like that. Or not disposing of wastes appropriately.